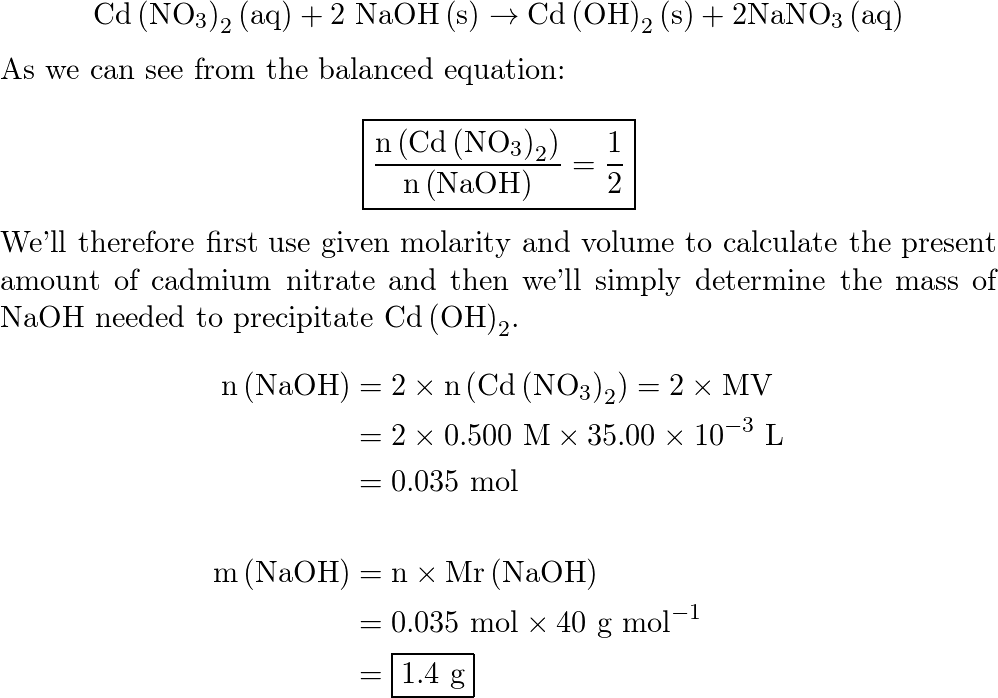Insights into the removal of Cd and Pb from aqueous solutions by NaOH–EtOH-modified biochar - ScienceDirect

SOLVED: What mass of NaOH is needed to precipitate the Cd2+ ions from 32.0 mL of 0.550 M Cd(NO3)2 solution?

Given the cell: `Cd(s)|Cd(OH)_2(s)|NaOH(aq,0.01M)|H_2(g,1bar)|Pt(s)` with `E_(cell)=0.0V.ifE_(Cd... - YouTube

Efficient removals of Hg and Cd in aqueous solution through NaOH-modified activated carbon fiber - ScienceDirect
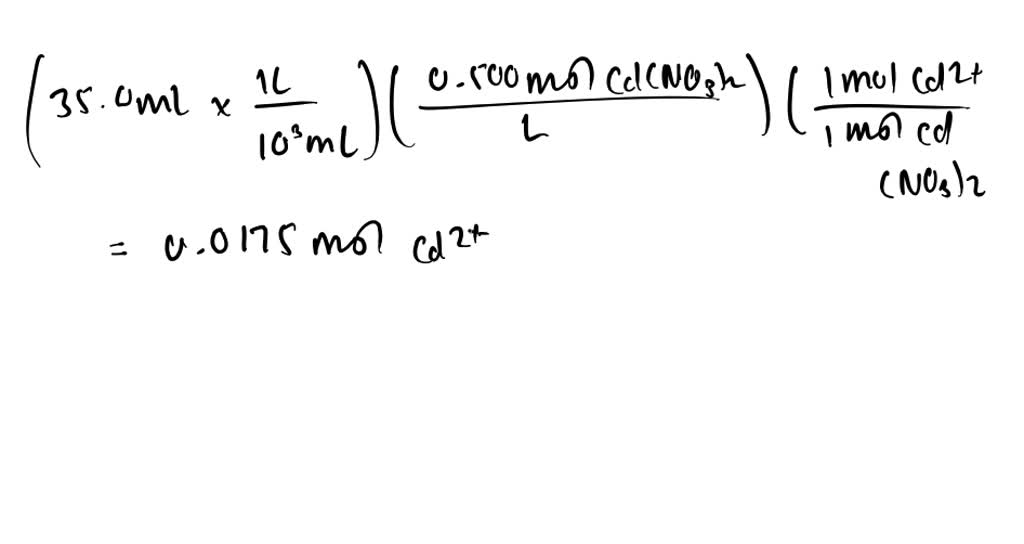
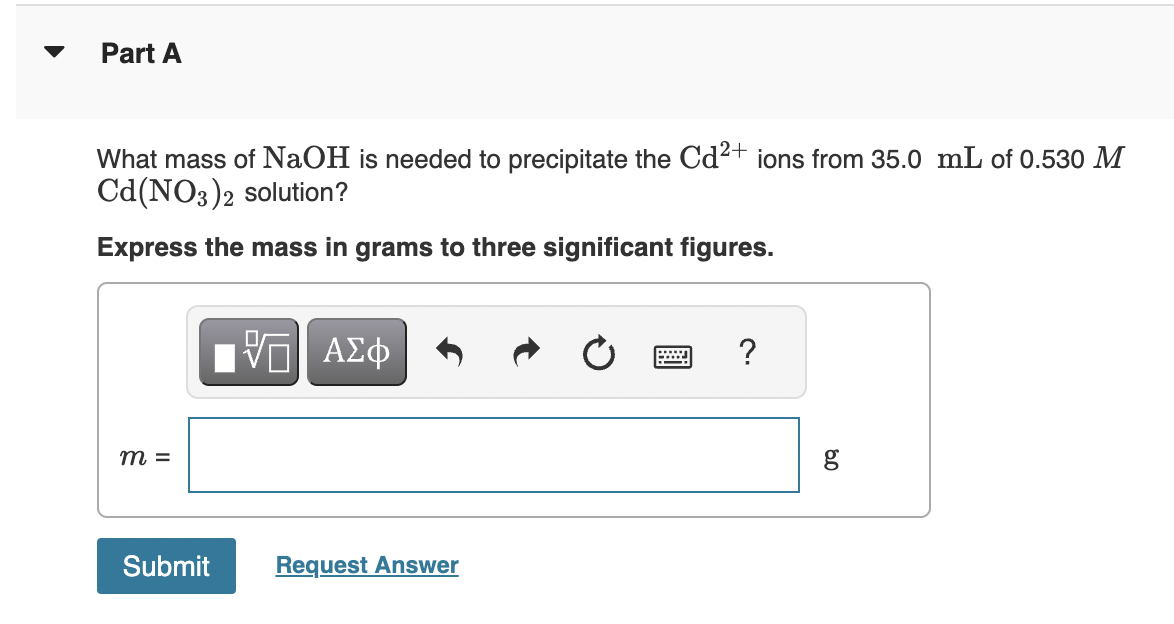
SOLVED:What mass of NaOH is needed to precipitate the Cd^2+ ions from 35.0 mL of 0.500 M Cd(NO3)2 solution?

Relationship between changes of extractability of Cd with NaOH and DTPA... | Download Scientific Diagram

Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:

Reduction of CD as a function of basic pH (Ca(OH)2 and NaOH) with a... | Download Scientific Diagram

Adsorption kinetics and isotherm of cadmium onto NaOH-treated oil palm empty fruit bunch - ScienceDirect

a) CD spectra for ligand 4 in basic media (2 equiv. of NaOH). (b) CD... | Download Scientific Diagram

Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:

Enhanced removal of aqueous Cd(II) by a biochar derived from salt-sealing pyrolysis coupled with NaOH treatment - ScienceDirect
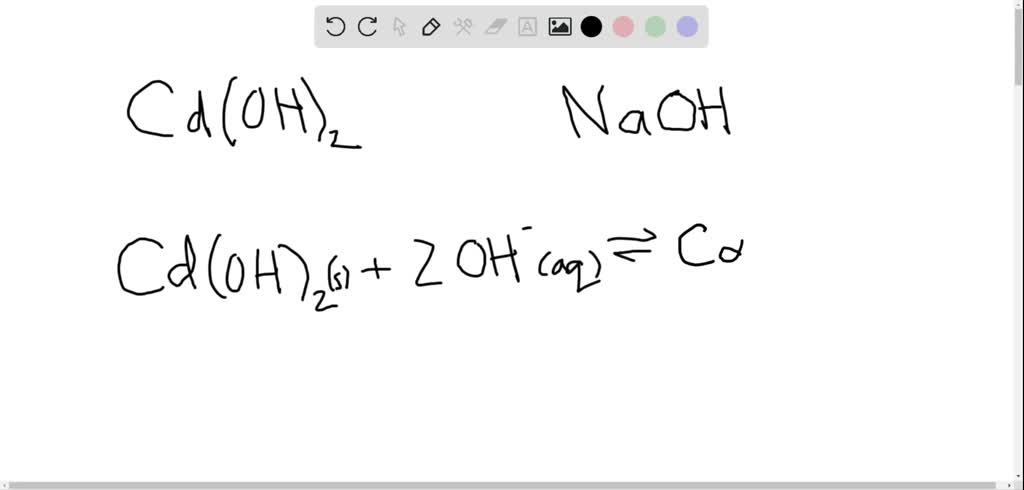
SOLVED:Cd(OH)2 is an insoluble compound. It dissolves in excess NaOH in solution. Write a balanced ionic equation for this reaction. What type of reaction is this?

Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:
![PDF] Effect of sodium hydroxide concentration on properties of carboxymethyl rice starch | Semantic Scholar PDF] Effect of sodium hydroxide concentration on properties of carboxymethyl rice starch | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7e887862fb34cbb37fc9fcd439d4ed28e875f2ed/6-Table3-1.png)

![ANSWERED] If 4 moles of NaOH react with 1 mole of C... - Physical Chemistry ANSWERED] If 4 moles of NaOH react with 1 mole of C... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/47412038-1658679986.4827924.jpeg)